Sunday, January 31, 2016
Saturday, January 30, 2016
Friday, January 29, 2016
Health Wonk Review is up at Managed Care Matters
Managed Care Matters has just just awoken from its post-party stupor after a wild celebration of the 10th anniversary of the Health Wonk Review. Maybe they should party more often because the writing is surprisingly crisp in the Tenth Anniversary Edition!
There are some great health policy posts from old-timers and newcomers alike. I’m proud to say I’ve been around since the very start.
Well done!
from Health Business Blog http://healthbusinessblog.com/2016/01/29/health-wonk-review-is-up-at-managed-care-matters-17/
via A Health Business Blog
Thursday, January 28, 2016
Like I said: United’s ACA exchange departure is no big deal

Robert Wood Johnson Foundation funded an Urban Institute study to determine how Obamacare marketplaces are being impacted by the withdrawal of United Healthcare and the failure of some of the CO-OP plans. I’m grateful for the data but as it turns out their findings line up with what I wrote more than two months ago (United pulls out of ACA exchanges: Should we care?)
Here’s what I said:
Recently we’ve heard what could be interpreted as bad news about the viability of exchanges:UnitedHealth is considering withdrawing from the program. In the highly politicized world of health reform, that information has Obamacare foes sounding the death knell.
I see things differently.
The big name insurers that have a large share of the corporate market are not necessarily the winners on the exchanges. Rather, the leaders in the new price-sensitive era are lesser known plans, many of which cut their teeth in the Medicaid managed care market where tight cost control is key. They have what it takes to play in this brave new world.
Compare that to the Urban Institute findings:
Co-ops and United Healthcare (United) exiting the marketplaces may not be the shake-up many predicted because Blue Cross insurers, managed care plans, and others dominate low-cost marketplace offerings.
- Blue Cross insurers and managed care organizations previously offering insurance to Medicaid beneficiaries but new to the private market are the most price-competitive.
- Blue Cross and Medicaid managed care organizations are among the lowest-cost insurers in 34 and 44 of the 81 regions, respectively.
Next time maybe I should apply for that grant rather than offering my instant analysis!
Image courtesy of Master isolated images at FreeDigitalPhotos.net
—
By healthcare business consultant David E. Williams, president of Health Business Group.
from Health Business Blog http://healthbusinessblog.com/2016/01/28/like-i-said-uniteds-aca-exchange-departure-is-no-big-deal/
via A Health Business Blog
Wednesday, January 27, 2016
Tuesday, January 26, 2016
Monday, January 25, 2016
Is Generally Regarded as Safe (GRAS) safe enough?

What did I eat?
Unlike most people exposed to the Frontline investigation of supplements and vitamins, I really didn’t find it shocking that a lot of supplements are dangerous and that the bottles don’t contain what they say they do. I feel sorry for people who take these products and are not helped –and are even harmed– but frankly the customers should know better.
The story got me thinking about a related topic: the safety (or lack thereof) of food additives. An NPR story from last year (Why the FDA has never looked at some of the additives in our food) sums it up well:
Companies have added thousands of ingredients to foods with little to no government oversight. That’s thanks to a loophole in a decades-old law that allows them to deem an additive to be “generally recognized as safe” — or GRAS — without the U.S. Food and Drug Administration’s blessing, or even its knowledge.
The loophole was originally intended to allow manufacturers of common ingredients like vinegar and table salt — when added to processed foods — to bypass the FDA’s lengthy safety-review process. But over time, companies have found that it’s far more efficient to take advantage of the exemption to get their products on shelves quickly. Some of these products contain additives that the FDA has found to pose dangers. And even ingredients the agency has agreed are GRAS are now drawing scrutiny from scientists and consumer groups that dispute their safety.
Basically companies have been declaring their own products as GRAS. Sometimes they let the FDA know and sometimes not. Since the GRAS concept was introduced during the Eisenhower administration the number of additives in food has gone from around 800 to over 10,000. Chances are you eat GRAS substances every day.
The FDA review process for food is cumbersome, which is a deterrent to undertaking it. But some safety testing is becoming radically less expensive and more reliable. One area I know about is cardiac safety, thanks to my role on the iCardiac Technologies board. Until recently, drugs in development were assessed for cardiac safety by a highly manual and expensive process of measuring QT prolongation. Now iCardiac’s Early Precision QT approach can provide definitive results with a much smaller and more affordable study and it is becoming the standard that is endorsed by FDA and international bodies.
So with the new approach, maybe it’s time to reconsider the cost/benefit of cardiac safety testing at least for certain food additives.
FDA’s “threshold of concern” is reached when a drug extends the QT interval by 10 milliseconds (ms). As it turns out, some food ingredients can reach or exceed this level. For example:
- An American Heart Association study found that energy drinks could boost QT by 10 ms.
- Licorice (also used as a sweetener in some products) can lead to long QT when “abused”
- Ginseng can increase QT by 15 ms.
Some of the more responsible companies like Cargill are undertaking safety studies for new products. Cargill has been evaluating a new sweetener (3000x as sweet as fructose!) derived from root bark. After some initial concerns were unearthed, the company conducted a QT study to determine the ingredient’s safety. As it turned out, this ingredient was found to cause a 20+ ms prolongation of the QT interval, which will certainly give Cargill pause before continuing development. Results of the study (Detection of ECG effects of (2R,4R)-monatin, a sweet flavored isomer of a component first identified in the root bark of the Sclerochitin ilicifolius plant) have been published in Food and Chemical Toxicology.
With new techniques, you don’t have to be Cargill to be able to afford to test food ingredients for safety. As consumers become more discerning it will become a good business decision to test more thorougly.
Image courtesy of David Castillo Dominici at FreeDigitalPhotos.ne
—
By healthcare business consultant David E. Williams, president of Health Business Group.
from Health Business Blog http://healthbusinessblog.com/2016/01/25/is-generally-regarded-as-safe-gras-safe-enough/
via A Health Business Blog
Sunday, January 24, 2016
Saturday, January 23, 2016
Friday, January 22, 2016
Thursday, January 21, 2016
Wednesday, January 20, 2016
Health Business Blog named as a top blog
The MHA@GW blog has posted a list of 54 Healthcare Blogs to read in 2016. It’s a solid collection of blogs from mainstream media, organizations and foundations, health care professionals, and other categories.
The Health Business Blog is featured alongside some of my favorites such as HealthBlawg, HealthPopuli, Not Running a Hospital and Workers’ Comp Insider.
from Health Business Blog http://healthbusinessblog.com/2016/01/20/health-business-blog-named-as-a-top-blog/
via A Health Business Blog
Tuesday, January 19, 2016
Sunday, January 17, 2016
Saturday, January 16, 2016
Friday, January 15, 2016
Evidence based defensive medicine

Defensive medicine –when physicians provide or recommend unnecessary treatment or testing in order to reduce their chance of being sued– has always bothered me. It harms the patient, drives up costs, and can be self-serving by generating more income for the provider. I’m also skeptical about whether “defensive” medicine really reduces the chances of being sued.
So I was very interested in a Today’s Hospitalist article (Does defensive medicine work after all?) that reports the results of an intriguing study of hospital admissions in Florida. The study, conducted by a Harvard Medical School professor, revealed that physicians who were responsible for the most expensive hospitalizations also had the lowest likelihood of being sued (0.3% vs. 1.5%).
There are plenty of limitations –correlation isn’t causation, it’s based on hospital admissions only, maybe the doctors in the high and low spending groups aren’t comparable, etc. — and yet it does give one pause. Maybe doctors who order more tests and treat patients more intensively really do get sued less. Could it be that patients and families are less likely to sue if they feel that everything has been done for them?
The findings have serious implications, especially as we leave the era of fee for service medicine and enter the age of accountable care and capitation. Will it be possible to get physicians to be less defensive in the name of cost savings? Is it fair to do so? What role should the patient and family have? Do we in fact need some kind of liability reform?
Dr. Anupam Jena, lead author notes that malpractice is bother under and over-stated. Malpractice costs are routinely reported at only 3-5 percent of total costs, yet physicians also say malpractice is a major concern. My own suspicion is that there’s only a limited correlation between real malpractice and what physicians actually get sued for.
Image courtesy of stockimages at FreeDigitalPhotos.net
—
By healthcare business consultant David E. Williams, president of Health Business Group.
from Health Business Blog http://healthbusinessblog.com/2016/01/15/evidence-based-defensive-medicine/
via A Health Business Blog
Thursday, January 14, 2016
Health Wonk Review is up at InsureBlog
Now that your New Year’s hangover has finally cleared, it’s time to head over to InsureBlog for its Happy New Year! edition of the Health Wonk Review. Hank has done himself proud with a stimulating array of wonky posts.
from Health Business Blog http://healthbusinessblog.com/2016/01/14/health-wonk-review-is-up-at-insureblog-16/
via A Health Business Blog
Wednesday, January 13, 2016
Tuesday, January 12, 2016
Monday, January 11, 2016
Sunday, January 10, 2016
Saturday, January 9, 2016
Friday, January 8, 2016
Thursday, January 7, 2016
Wednesday, January 6, 2016
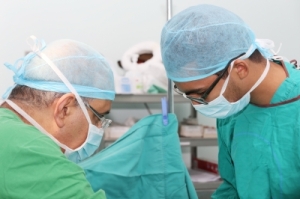
Free market for surgery: interview with Allevian CEO Arnon Krongrad, MD
Surgery can be expensive, scary, dangerous and even deadly. Yet it’s hard for patients and even for referring physicians to navigate the system. So I was intrigued when I was contacted by Dr. Arnon Krongrad , CEO of Allevian, Inc., a healthcare logistics company that markets surgery packages. The company’s Surgeo online marketplace let’s patients shop for the surgeon of their choice.
I explored the topic in depth with Dr. Krongrad in this email interview.
What are the limitations of referrals to surgeons by primary doctors?
Conventional surgeon referrals by other doctors, such as primary physicians, rely upon limited knowledge about a limited number of surgeons. For example, primary physicians tend to know a relatively small handful of surgeons for specific procedures, such as vision correction. The simple reality is that in today’s environment, when primary doctors are pressured to churn patients, type into EMRs, and speak ICD-10, they have little time to sort through choices of surgeons. They will reflexively refer to the familiar, which may or may not be optimal. Primary physicians, like the rest of us, might welcome systematic, easy access to knowledge about choice of surgeons.
It looks like your surgeons are selected based on the opinions of their peers. Are there more objective, quantitative ways to rate surgeons?
Surgeo’s purpose is to simplify access to quality care. In surgery, quality comes from the surgeon. The question, then, is how to qualify surgeons. This is an urgent question as our increasingly consumerist ecosystem demands transparency regarding cost and quality. It is a question with obvious and easy answer but a conceptual framework will help us to manage and make progress.
First of all, no, there are no objective, quantitative ways to rate surgeons. We should be clear. We are not talking here about waiting room times or adequacy of parking. We are talking here about clinical quality: blood loss, rectal perforation, positive margins, and the like. A system that has not produced an objective, quantitative way to transparently, uniformly, and broadly present cost – a much more cleanly quantifiable dimension of care – has only just begun to think about objective, quantitative ways to present surgeon quality.
As we think about surgeon quality, we should recognize that surgeons do not operate in a vacuum. Most critically, they operate on patients, who are varied, and produce outcomes that are varied. Let us use prostate cancer surgery as an example. The factors other than surgeon skill that affect various outcomes vary. For example, cure is affected by cancer grade and preservation of erections is affected by diabetes. So which outcome – cure, erections – are we looking at when we ask about surgeon quality? Once we decide, have we collected patient data in a way that would permit us to explain variation in outcomes between surgeon A and surgeon B? No, we’re just not there yet.
Secondly, formulaic objectivity, laudable as it might be, is not the only way to go. The other way is subjectivity, the argument for which was previously laid out in this article. In brief, the principle relates to the instantaneous ability of subject experts to detect artistry when they see it. It argues that surgeons know quality surgery when they see it. As one very senior surgeon who has taught surgery all around the world said it: “I can tell within a minute of skin incision if a surgeon is good.”
There are data, most notably in bariatric surgery, to show that subject expert peer credentialing correlates with objectively measured outcomes, such as hospital readmission. In other words, we think we can do well by patients and payers by applying the collective, subjective wisdom of surgeons.
Do other factors affect surgeon qualification? Any surprises?
Yes. Surgeo’s surgeons are qualified using a published, multi-factorial surgeon credentialing process that includes but is not limited to peer input. The idea is to try to get as close as possible to surgeons who know what they are doing and who exemplify the high standards of service and mission.
The additional steps have yielded some surprises. For example, Surgeo recently rejected the application of a very well known, very high volume, well published surgical specialist whose peers think highly of him. We rejected the application upon discovering what his peers did not know: that he has a history of numerous high-dollar malpractice payouts, including one incomprehensible loss at jury trial. This example relates to the opening comments: doctors do not investigate surgeon qualifications as fully as they might if given motivation and time. Surgeon qualification takes time, effort, and more than one criterion.
What do surgeons think of your approach?
Doctors generally are under siege. They are all looking for simplicity, fairness, and respect for their abilities and integrity. Surgeo delivers those and surgeons love the surgeon driven, clinically rational product design and administrative approach. The model works because it satisfies patients and providers. It has also satisfied payers, as when we used it in a previous chapter to satisfy Blue Cross and develop surgery bundles for the network.
What has been the most surprising reaction of your surgeons?
Surgeons are often paralyzed by the freedom that Surgeo offers. They have no idea how much to charge for their services because nobody has ever asked them before. Surgeo does not negotiate prices with its downstream vendors. Surgeo does not set allowed amounts or ask for discounts. This is unfamiliar to most doctors. This seems bizarre. Have you ever met a lawyer who has no idea what his hourly rate is?
What’s the history of Surgeo? What is its future?
One day, two things happened: Congress said everyone was getting healthcare and a man with cancer told me he could not get healthcare: surgery for his newly diagnosed cancer.
I tried to help him and ran into inflexibility and apathy. The solution ultimately involving sending him from Oregon, my surgical team from Florida, and a surgical robot spot purchased on eBay for 1% of retail from Colorado to an operating room in Trinidad. The exercise delivered a flat-fee surgery package at a price he could afford and worked for all involved. We then developed the model domestically and sold surgery packages to individuals and large payers. You can see a presentation of that case here.
Surgeo is a public, interactive, online face of surgery packaging and pricing software engine that was designed in-house under the direction of Kimberly Langer, our Chief Product Officer. Kim was formerly with a large payer, where she designed large enterprise claims related software. She built Surgeo to scale in a way that can work with payer EDI streams for easier network integration and for presentation to members of service choices and out-of-pocket costs. Kim also built it to accommodate our customers who want privately labeled software by which to market their own surgery packages. We’re seeing demand for that from financial and provider organizations.
You cover a variety of surgeries. Are there any surgeries that have been more popular than expected?
Penile implant surgery. We did not even think about this when we first set out. It turns out that there are huge problems related to this procedure. First of all, authentic conversation about male sexuality and erectile function is in very short supply and men with diabetes, cancer, and other conditions associated with erectile dysfunction have very few places to turn for substantive learning. Secondly, the pharmaceuticals for erectile dysfunction have taken over the airwaves and displaced much of the conversation about other, very effective treatments. We were amazed, for example, that one national network of diabetes activists, whose constituents have up to 70% prevalence of erectile dysfunction, has not discussed erectile dysfunction in 10 years!
What makes the challenges even greater is that penile implant surgery is often not covered by payers. We hear regularly from patients who thought they had what they call “really good insurance” that penile implant surgery is not covered. The way things are going, with CMS having dropped covered for vacuum erection devices, it won’t surprise us if penile implant surgery is just uniformly dropped.
We are getting sad, pleading calls from men with diabetes, obesity, cancer, and others who would like to restore their erections, relationships, marriages, and mood. These men are finding a delivery system that is broadly opaque and unhelpful. In response, we built in response is a national penile implant surgery package network. It features peer credentialed surgeons, comprehensive flat-fee packages, finance navigation, and financial protection in the event of complications. It offers plenty of choice.
The risk protected, flat-fee penile implant surgery package network is a model for efficient delivery of non-covered services. It can help payers to help direct members who do need those services.
What do you mean by financial protection in the event of complications?
Surgeo packages protect surgical patients against financial surprises by bringing in qualified surgeons. This is not a guarantee of elimination of complications but it helps. The second protection is inclusion in the flat-fee package of ancillary procedures. For example, gastric sleeve surgery includes hiatus hernia repair if it is needed: no surprise bill. In some cases, such as penile implant and laparoscopic hysterectomy, we are able to also include third-party products that take financial responsibility in the event of surgical complications. Think of it like getting collision insurance when renting a car.
What explains the variations in package prices?
We are just now starting to see a geographically distributed free market of uniformly defined surgery packages. Surgeons in Houston are looking and seeing prices in Birmingham and adjusting accordingly. So some of the variation is explained by the absence of a transparent market. Variation will probably shrink as price transparency sets in as it has on Surgeo. We see surgeons routinely not wanting to the most expensive.
Image courtesy of David Castillo Dominici at FreeDigitalPhotos.net
—
By healthcare business consultant David E. Williams, president of Health Business Group.
from Health Business Blog http://healthbusinessblog.com/2016/01/06/free-market-for-surgery-interview-with-allevian-ceo-arnon-krongrad-md/
via A Health Business Blog
Tuesday, January 5, 2016
Monday, January 4, 2016
How crazy is Ted Cruz’s FDA reform proposal?
I don’t think very highly of Senator and Republican Presidential candidate Ted Cruz, but his proposal to loosen the drug approval process is at least worth discussing. If you haven’t heard, Cruz’s RESULT Act proposal is as follows:
- FDA to grant reciprocal approval of “life-saving” drugs and devices from regulatory agencies in developed countries including EU, Israel, Australia, Canada and Japan. FDA would have only 30 days to review and approve
- Congress can override FDA decisions with a majority vote
Basically he is trying to make it easier to get drugs and devices on the market. There are a number of problems with this approach, which are pointed out by my most of the respectable analysts. Objections include:
- FDA may just reject the applications anyway since 30 days is not a long enough time to review and they may object to procedures used elsewhere
- Insertinbg Congress directly into the process undermines the scientific basis of the decision
- Trial sponsors may seek the jurisdiction with the lowest standards or fastest review times, imperiling safety for Americans
- It would hurt US economic development by shifting development resources overseas
- We would be ceding our sovereignty to foreigners
- FDA is already pretty fast and responsive and has programs for compassionate use and acceleration of the approval process when warranted
These are all reasonable, and yet I was struck by the fact that almost all the commentators use the Thalidomide debacle as their one and only example. See for example STAT, The FDA Group Blog, and Harvard Law Blog, which are the top articles that come up under a Google search for “Ted Cruz FDA proposal.”
Thalidomide was approved in Europe as a sleeping pill and for morning sickness but rejected in the US. It caused serious birth defects, with thousands of people affected in Europe but not the US. Great example but we’re talking 1956. Remember 1956? I don’t. But to give you some perspective it was just two years after food rationing was lifted in the UK and nine years before Medicare was established. I don’t hear people citing 1956 examples about anything else in healthcare.
A more serious discussion could be held on the balance between safety and efficacy in FDA approvals. Cruz’s impulses might be better directed to that debate. Maybe it’s ok for FDA to approve any product that’s safe, and not force sponsors to prove efficacy. Sponsors would still want to demonstrate efficacy, but they’d need to do so for healthcare payers, not the FDA.
That’s not as simple as it sounds either. Here are some of the challenges:
- The biggest payers are Medicare and Medicaid, so even if you take FDA out of the business of judging efficacy the government is still involved
- In many situations, safety is relative, not absolute. I might accept a modestly effective cancer drug that kills 1 percent of those who take it, while I wouldn’t accept a cure for toenail fungus with the same death rate. But it’s also important to protect cancer patients from dangerous drugs. A recent NYT article about the death from cancer of the wife of the FDA’s head of oncology included this important and under-appreciated point: “Cancer medicines not only often fail to save patients but can accelerate their deaths and make their last weeks far more painful”
- Safety and efficacy are individualized. (Cruz seems to recognize this.) Some drugs will be effective for only a small population; similarly safety issues don’t always apply across the board. I would want access to a drug that could cure me even if it could kill someone else, especially if there were a companion diagnostic to sort out who will be helped and hurt
Overall I think the FDA is doing a good job of managing the conflicting pressures it faces. Some parts of FDA (such as the groups with responsibility for HIV and oncology) do a better job than others.
I really would like to see Cruz’s proposal spur a more productive debate and not simply be dismissed out of hand.
Image courtesy of vectorolie at FreeDigitalPhotos.net
—
By healthcare business consultant David E. Williams, president of Health Business Group.
from Health Business Blog http://healthbusinessblog.com/2016/01/04/how-crazy-is-ted-cruzs-fda-reform-proposal/
via A Health Business Blog